Medical Device Operation License Application
Setting up medical device companies in China is becoming more common. Apply for a medical device operation license and start your business!
Requirements for Applying for Medical Device Operation License
In Hongda’s previous blog, we introduced the essential licenses to run your food operation business in China (click here to read more).
On this service page, Hongda will guide you to a deeper understanding of when you, as a participant in the food business industry, need to apply for a Food Business License or Food Production License. Once your specific application needs are confirmed, Hongda will be willing to assist you in obtaining the necessary documents!
- Covering ‘operation of medical devices’ in the company’s business scopes: An application for the operation of medical devices must be submitted based on a company entity whose main business scope focuses on the operation of medical devices. Ensuring a related and comprehensive business scope should be planned as you register your WFOE in China.
- Meeting the compulsory requirements of different classifications: According to China’s industry standards, the operation of medical devices can be further divided into three classes (Class I, Class II, and Class III) with different requirements. Different types of medical devices may fall into different classes where application materials and requirements vary.
- Ensuring compliance with practical operating conditions: In other words, the place where you will be storing your medical devices or running your operation must meet the requirements for public supervision and inspection purposes. For example, for a "Class III License," a qualified warehouse is mandatory. The office space should not be less than 60 square meters, with a minimum of 5 employees (including at least 1 professional with previous background in the medical device field) and equipped with dedicated medical device management software.
Comparison of the Differences between Three Classes of Medical Device Operation Licenses
Class I
Class II
Class III
Scope of Application
Low-risk medical devices
High-risk medical devices
Description
These devices have low risk and typically include non-invasive, low-risk products.
Devices in this category pose a moderate level of risk and include products that require invasion into the body, with higher associated risks.
These devices are considered high-risk products, often involving complex technologies and invasive procedures.
Examples of instruments
Surgical instruments, X-ray films, surgical gowns, surgical caps, examination gloves, gauze bandages, drainage bags, regular hospital beds, wheelchairs, etc.
Adhesive bandages, condoms, thermometers, blood pressure monitors, oxygen concentrators, nebulizers, etc.
Infusion sets, syringes, intravenous catheters, cardiac stents, ventilators, CT scanners, magnetic resonance imaging (MRI), pacemakers, artificial heart valves, artificial joints, etc.
Application method
No additional application is required.
Submit a record application for approval to the municipal department responsible for drug supervision and management.
Submit an application to the municipal Administration for Market Regulation in your city (on-site inspection is required).
Personnel Requirements
Basic qualifications for key personnel only.
At least 1 professional with expertise in medical devices for wholesale enterprises dealing with no more than 8 categories of products.
At least 2 full-time quality management personnel are required, with at least 1 person possessing expertise in medical devices for enterprises dealing with more than 8 categories of products.
Over 5 employees, with at least 1 person possessing a professional educational background in medical devices or a related field*.
Additional requirements need to be met for specific categories of medical devices, such as contact lenses, IVD, and invasive and implantable medical devices.
If the intended employee does not pass the probationary period, a new candidate will be provided.
* Professional expertise in medical devices includes fields such as medical devices, biomedical engineering, mechanical engineering, electronics, medicine, bioengineering, chemistry, pharmacy, nursing, rehabilitation, laboratory medicine, management, and related disciplines.
Application of Medical Device Operation License Step-by-Step
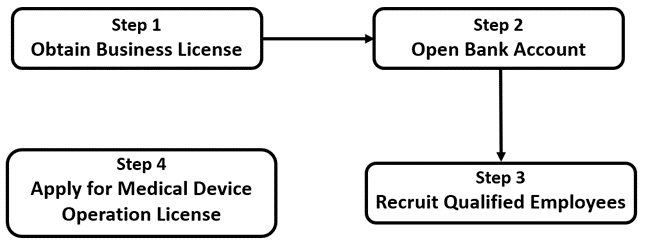
- Establish a company as the business entity (usually a WFOE) and obtain a business license: If you want to engage in the medical device operation industry, you need to register a company as the main business entity, covering the item 'medical device operation’ in your operation scope. Dingxin will help you through this process and provide all necessary assistance so that you don’t need to worry about the compliance requirements about determining your business scope!
- Open a bank account and register for taxation: Dingxin can also help you keep your financial records and report your taxes with our bookkeeping services. Please visit our bookkeeping service page for more information!
- Set up an office and warehouse: The government has set regulations on the standards of the office and operation areas of any medical device companies, including location, sizes, fire controls, and other criteria. Make sure your ideal location meets all the requirements, or let Dingxin help you!
- Recruit employees: Due to the nature that the medical device industry involves high-tech operation and understanding of complex technical indicators, which requires comprehensive knowledge within this field, the educational and professional qualifications of the employees need to be assessed and examined according to related laws and regulations. Dingxin can also help you with this!
- Apply for a Medical Device Operation License: Prepare all the materials and documents needed and submit the application for your Medical Device Operation License to be approved! If the business involves cross-border trades of medical devices, an Import and Export License is also required. But no worries, as Dingxin will help you with all these!
Already Have Your Company for Application?
Step One: Understand Relevant Laws and Regulations
Before applying for a Medical Device Operation License, it is crucial to have a solid understanding of the relevant laws and regulations. Familiarity with these regulations is essential to avoid errors during the license application process. This knowledge can be acquired through the official website of the National Medical Products Administration or other regulation inquiry websites. The easiest way to do so, however, is to work with Dingxin and let us tell you everything you need to know!
Step Two: Qualification Review Application
After gaining knowledge of the applicable laws and regulations, the next step is to prepare the necessary documentation for a qualification review. This review is conducted to verify whether the applicant meets the conditions for operating medical devices. Required documents include the business license, organization code certificate, tax registration certificate, and the legal representative's identification, along with other supporting materials.
Step Three: Submission of Application Materials
Submitting the application materials is a critical step in obtaining a Medical Device Operation License. Before submission, it is essential to thoroughly understand the relevant regulations and the specific documentation required. Application materials typically include a business plan, a catalog of medical device products, quality management and financial system documentation, and proof of qualifications for relevant personnel. Dingxin will help you all the way through with our ‘no success, no charge’ promise. Just relax!
Step Four: On-Site Assessment
Following the submission of application materials, the government will conduct an on-site assessment of the company. This assessment covers aspects such as warehouse size, equipment, office staff numbers, and an evaluation of the product quality management system.
Step Five: Approval!
After the on-site assessment, the application undergoes an approval process. If approved, the company will receive the Medical Device Operation License. In the event of non-approval, addressing the reasons for rejection is necessary, and the application may need to be resubmitted.
Frequently Asked Questions
Still have a question? No worries! We are glad to answer!
What should I do with the license if my enterprise no longer engages in the business of medical devices?
If your enterprise voluntarily terminates the business of medical devices, you shall apply to the original issuing department for the cancellation of the "Medical Device Business License". You are required to submit the cancellation application and relevant supporting materials, such as the statement that the inventory has been cleared, etc. After being approved by the original issuing department, the license will be cancelled and the corresponding business termination procedures will be completed.
What are the requirements for personnel when I apply for the medical device business license?
Different categories of medical devices have different requirements. For Class III medical devices, your enterprise needs a quality management organization or qualified personnel with relevant professional degrees or titles. Staff in acceptance, etc. should have corresponding knowledge and capabilities. For in vitro diagnostic reagents, relevant staff need a lab medicine background.
What are the regulations on the business premises and warehouses for my enterprise?
Your business premises and warehouses must fit the business scope and scale. Warehouses should meet storage requirements, have necessary facilities like light-proofing ones, and for those with special storage needs (e.g., cold chain products), proper conditions must be provided. The warehouse area for Class III implantable medical devices should meet inventory storage needs.
What are the essential requirements for my enterprise's quality management system?
Your enterprise should establish and maintain an effective quality management system covering procurement, acceptance, storage, sales, transportation and after-sales service, ensuring the traceability of medical device quality and safety, like having an incoming inspection record system.
Please give us a message
Tel:15066768857
E-mail:sales@dxjclegalconsulting.com
